
Your clinical trial software solution

The clinical trial recruitment process is better with Evrima
Easy participant registration
Trial participants can register their interest and provide additional information in the secure participant portal and have visibility on their clinical trial progression.
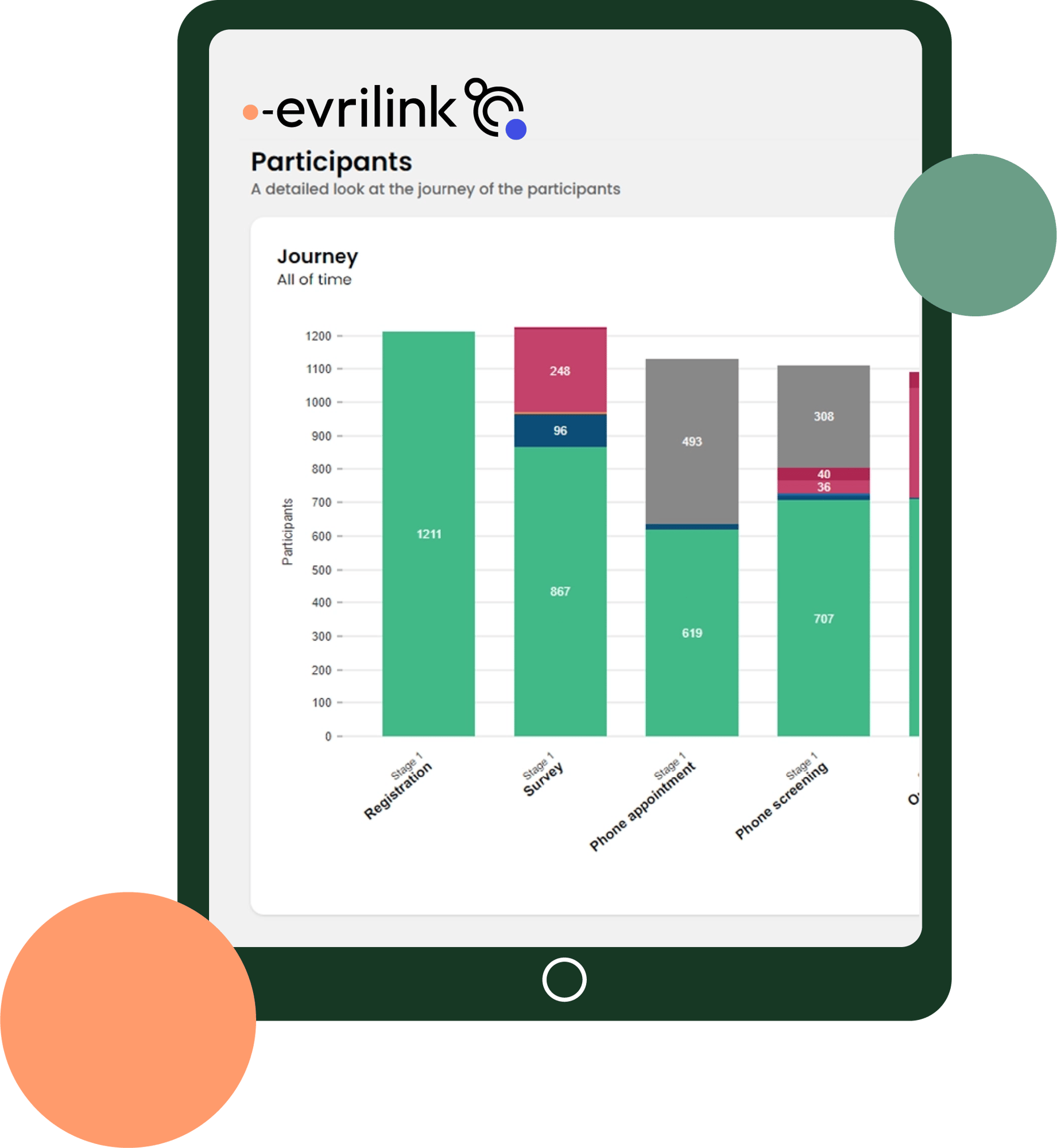
Real-time recruitment data
Sponsors and research organisations are empowered to make data-driven decisions. Evrilink delivers a single source of truth for clinical trial recruitment progress.
A secure portal
Evrima’s advanced data privacy and data protection solutions means participants, trial sponsors and clinical trial management teams alike, are protected.
Better communication & reporting
Email notifications, in-platform communication and enterprise reporting are just some of the features that are built into the easy-to-use Evrilink platform.
They trust us
90%
A report from the Clinical Trial Action Group released by the Australian Government revealed that 90% of trials in Australia experience recruitment delays. Evrima is working to change this statistic. Now.
86%
It’s a global challenge. 86% of all clinical trials in the United States, and 69% of trials in the United Kingdom, also fail to achieve recruitment targets on time. Let’s change this.
Evrilink in action
Request a demoLearn how Evrilink is delivering a better clinical trial process - for everybody. It’s patient-focused, customisable, proven technology.
Global leaders in the clinical trial sector are choosing to partner with Evrima for better recruitment and management solutions.
Designed to deliver an optimum clinical trial experience
Evrilink responds to specific clinical trial recruitment and management challenges - with specific solutions.
The customisable platform is designed and built to provide a better user experience for everybody; patients, trial participants, clinical trial sponsors and trial site managers alike. Every clinical trial is different. Customise the Evrilink platform around your needs and expedite your clinical trial process.
Better features
Enterprise reporting capabilities
Gain a holistic view of recruitment and enrollment progress with real-time, de-identified reporting. Use date, stage, site and location filters.
Site participant database management
Make your participant database work harder. Seamlessly upload, manage and invite participants to clinical trials.
Easy registration
Participants can register their interest, upload documents, complete screening surveys and view their registration progress, 24/7.
Better visibility
Clinical trial sponsors, CROs and sites can access real-time trial recruitment data and have full visibility of participant progress, 24/7.
In-platform communication
Streamline communication with your fellow clinical trial colleagues by commenting or tagging members of the team.
Secure data
Advanced privacy and data protection safeguards are built in. We’re Good Clinical Practice and 21 CFR Part 11 compliant and have a robust Privacy Policy and Terms of Service in place.
Multi-site management
Evrilink is a scalable platform that can be quickly deployed across single or multiple clinical trial sites.
Easy to implement
It’s quick and easy to get started with Evrilink. It’s an intuitive platform, with minimal training required. The Evrima team is here to help.
Evrilink FAQs and answers
01
How does Evrilink streamline my communication with participants?
-
Evrilink automates communication with clinical trial participants so they are kept informed when they are referred, withdrawn or disqualified from the recruitment process. Better communication, delivers a better clinical trial experience for the participants and reduces the workload of clinical trial teams.
02
How does Evrilink streamline my communication with clinical trial Sponsors?
+
Sites and Sponsors have real-time access to enterprise level reporting so they are able to access recruitment progress when they need to. Clinical trial sites no longer need to provide pre-screening logs in email or spreadsheets, and Sponsors or CROs no longer need to collate multiple sources of data across their different sites.
03
How do clinical trial sites use Evrilink?
+
Clinical Trial Sites can use Evrilink to manage their participant screening and recruitment for each trial. Sites can also use Evrilink to manage and maximise the potential of their own participant database.
04
What types of clinical trials can Evrilink support?
+
Evrilink supports phase 1 to 4 clinical trials across many different therapeutic areas. If you have questions about your specific clinical trial, contact us, we’re here to help.
Latest news
No Results Found
Featured resources


